 Exploring shapes
Exploring shapes
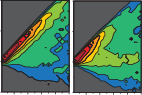
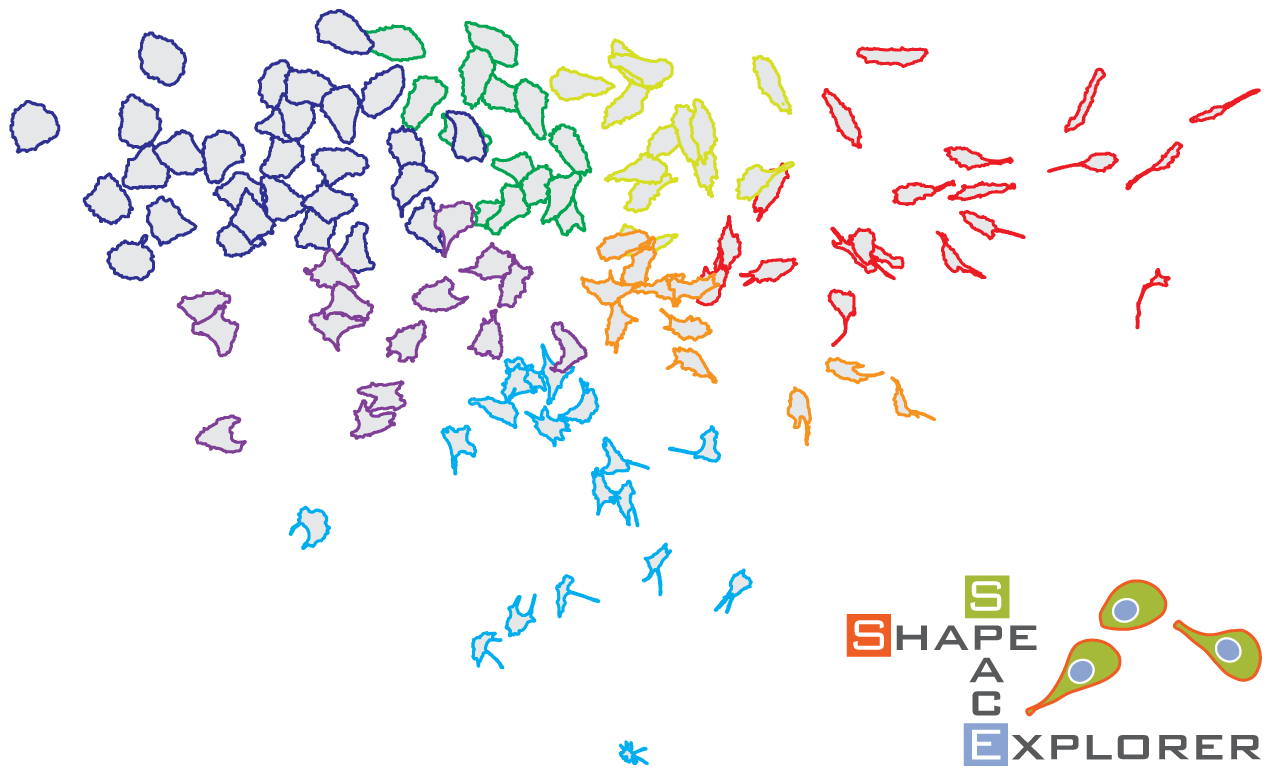
We describe the development of ShapeSpaceExplorer, an interactive software package that makes it easy to analyse cell morphology without any prior knowledge of relevant shape features and in particular enables tracking how cell shape changes dynamically. At the core of our software is a new and efficient method to measure the similarity of two shapes. Pairwise distances from all shapes in the dataset are used to position each shape in a low-dimensional map of shape space. The user can explore this shape space and visualise average shapes from any region of interest, partition shape space to analyse shape distributions from different experimental conditions or measure the speed of shape changes between two regions of shape space. We also show that shape dynamics information alone can be used to predict when migrating cells change direction.
Jefferyes, S.D.R., Gostner, R., Cooper, L., Abdelsamea, M.M., Straube, E., Rajpoot, N., Epstein, D.B.A. and Straube, A.* (2026)
ShapeSpaceExplorer: Analysis of morphological transitions in migrating cells using similarity-based shape space mapping.
PLoS Computational Biology, 22(1): e1013864, doi: 10.1371/journal.pcbi.1013864
[Link to open access paper]
PREPRINT bioRxiv 2025.08.19.671051
[Link to preprint]
Software, documentation and datasets
[Link to documentation] [Link to code] [Link to image data] [Link to processed datasets]
 motor co-dependence mechanisms
motor co-dependence mechanisms
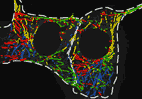
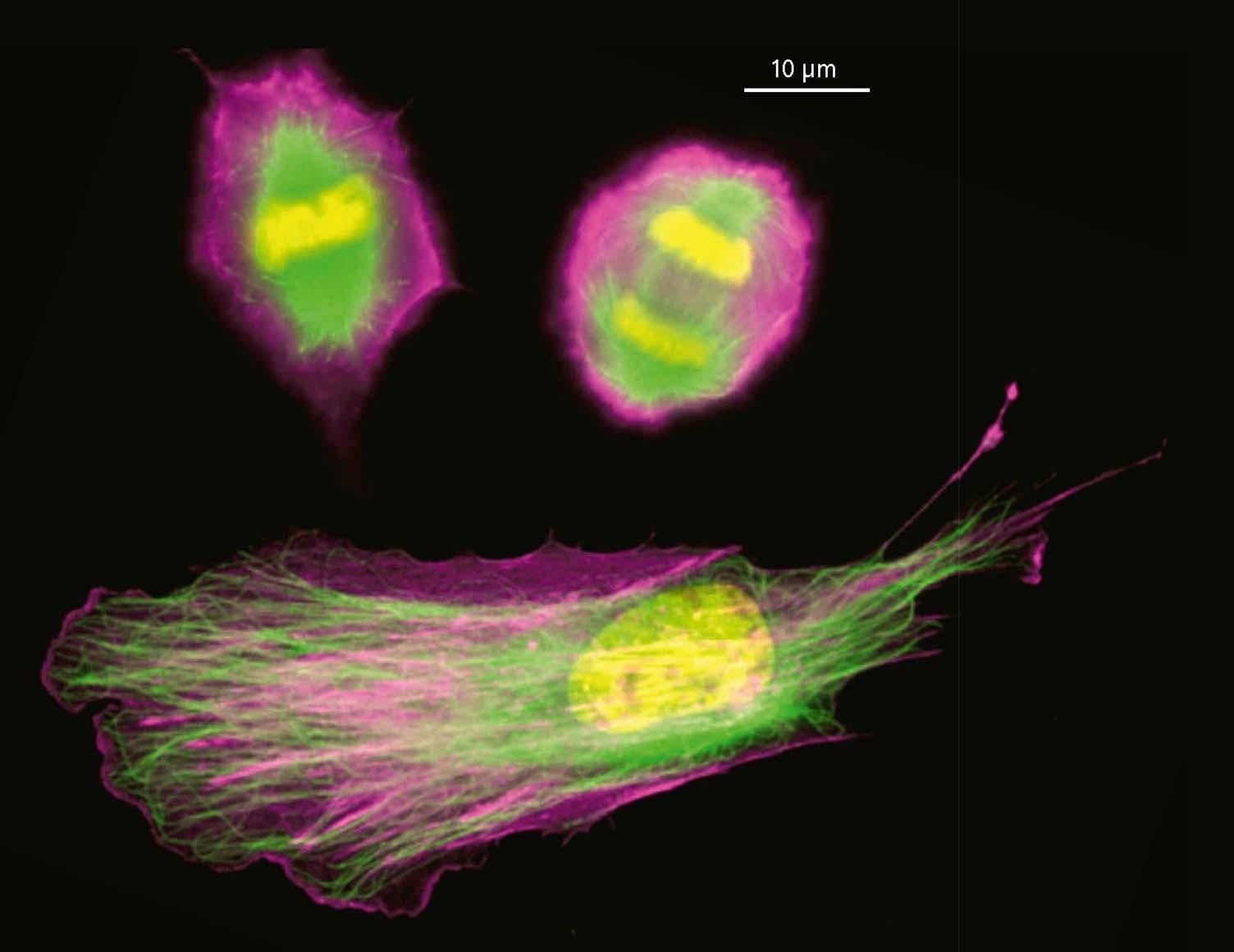
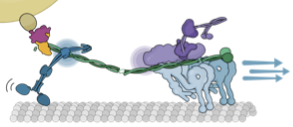
We show that complexes of dynein, KIF1C, dynactin and cargo adapter Hook3 undertake plus and minus end-directed motility along microtubules. Both motors activate each other through the steric disinhibition of the cargo adapter. In addition, KIF1C extends dynein-mediated runs by acting as a weak microtubule tether. This study reveals a mechanistic understanding of co-dependence of opposite polarity motors.
Abid Ali, F., Zwetsloot, A.J., Stone, C.E., Morgan, T.E., Wademan, R.F., Carter, A.P.* and Straube, A.* (2025)
KIF1C activates and extends dynein movement through the FHF cargo adaptor
Nature Structural and Molecular Biology 32, 756–766, doi: 10.1038/s41594-024-01418-z
[Link to open access paper]
Cover image of the issue [Link to Cover]
Accompanying News&Views article by Steven Markus in the same issue [Link to News&Views article]
PREPRINT bioRxiv 2023.10.26.564242v1
[Link to preprint]
 surfing the end
surfing the end
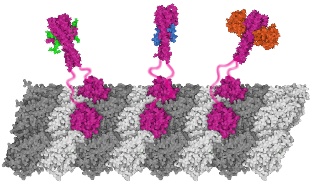
We describe an analytical mathematical model that demonstrates how processive motility is enabled by preferential binding and unbinding of cargo-substrate links. Thereby a cargo can track newly generated or progressively disappearing binding sites such as the tip of growing or shrinking microtubules.
Mosby, L.S., Straube, A. & Polin, M. (2023)
A general model for the motion of multivalent cargo interacting with substrates.
J. R. Soc. Interface 20: 20230510.
[Link to paper]
 weak motors cause neuronal disease
weak motors cause neuronal disease
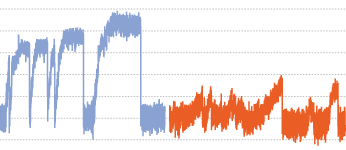
We report that KIF1C is a strong processive motor with a stall force of 5.7 pN and characterise two pathogenic mutations P176L and R169W that cause hereditary spastic paraplegia and cerebellar dysfunction. Both mutations reduce microtubule binding, but are fast and processive motors in single molecule assays. However, their force generation is severely impaired and they cannot efficiently relocate cellular cargo even if working in teams.
Siddiqui, N., Roth, D., Toleikis, A., Zwetsloot, A.J., Cross, R.A. & Straube, A. (2022)
Force generation of KIF1C is impaired by pathogenic mutations
Current Biology 32(17): 3862-3870.e6
[Link to paper]
DESPATCH by Will Hancock in the same issue [Link to despatch]
SSRN 10.2139/ssrn.4075230 [Link to sneak peak]
PREPRINT bioRxiv 10.1101/2021.06.30.450611 [Link to bioRxiv preprint]
 end binding proteins
end binding proteins
Our contribution to the third edition of the Encyclopedia of Biological Chemistry is a further reading section on microtubule plus and minus end binding proteins.
Mosby, L.S. & Straube, A. (2021)
Further Reading | Microtubule Plus and Minus End Binding Proteins
Encyclopedia of Biological Chemistry III (Third Edition), Elsevier, pages 554-566,ISBN 9780128220405,
[Link]
 endometrial pericyte migration
endometrial pericyte migration
A collaboration with clinical scientists interested in endometrial mesenchymal stem cells. Here we show that vascular edhesion protein-1 (VAP-1) is required for proper cell migration, adhesion, contractility and clonogenic behaviour.
Gharanei S, Fishwick K, Peter Durairaj R, Jin T, Siamantouras E, Liu K-K, Straube A, Lucas ES, Weston CJ, Rantakari P, Salmi M, Jalkanen S, Brosens JJ and Tan BK
(2021)
Vascular Adhesion Protein-1 Determines the Cellular Properties of Endometrial Pericytes.
Front. Cell Dev. Biol. 8:621016.
doi: 10.3389/fcell.2020.621016
[Link]
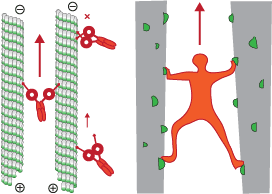
 kinesin-3 long distance transporters
kinesin-3 long distance transporters
Our chapter in the kinesin superfamily handbook describes the largest subfamily, kinesin-3, a group of motors recently shown to be superprocessive and working as cellular long-distance transporters.
Nida Siddiqui and Anne Straube (2020)
The Kinesin-3 Family: Long-Distance Transporters
in: The Kinesin Superfamily Handbook: Transporter, Creator, Destroyer, ed. Claire T. Friel, CRC Press, 2020
[Link to Book] [Open Access Chapter]
 rapid test for SARS-CoV-2
rapid test for SARS-CoV-2
Our colleagues in the Chemistry department at Warwick developed a rapid test for SARS-CoV-2 based on the affinity of spike protein to glycans. We purified spike S1 protein from HEK cells for testing the device.
Alexander N. Baker, Sarah-Jane Richards, Collette S. Guy, Thomas R. Congdon, Muhammad Hasan, Alexander J. Zwetsloot, Angelo Gallo, Józef R. Lewandowski, Phillip J. Stansfeld, Anne Straube, Marc Walker, Simona Chessa, Giulia Pergolizzi, Simone Dedola, Robert A. Field, and Matthew I. Gibson (2020)
The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device.
ACS Central Science 6(11):2046-2052 doi: 10.1021/acscentsci.0c00855
[Link to Open Access Paper]
Alexander N. Baker, Sarah-Jane Richards, Collette S. Guy, Thomas R. Congdon, Muhammad Hasan, Alexander J. Zwetsloot, Anne Straube, Marc Walker, Simona Chessa, Giulia Pergolizzi, Simone Dedola, Robert Field, and Matthew Gibson (2020)
The SARS-COV-2 Spike Protein Binds Sialic Acids, and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device.
PREPRINT chemRxiv 12465680
doi: 10.26434/chemrxiv.12465680 [Link to Preprint]
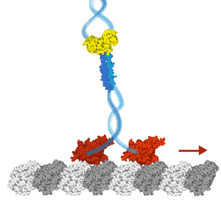
 dynein activation on microtubule bundles
dynein activation on microtubule bundles
We show that human dynein is activated when it crossbridges two microtubules. Dynein polarity-sorts microtubule bundles by selectively sliding anti-parallel microtubules apart. While single molecules of dynein are predominantly static or diffusive on single microtubules, they walks processively on the microtubule bundles they form. Their force output doubles on microtubule bundles and we propose a model whereby the separation of the motor domains during crossbridging activates the motor.
Manas Chakraborty, Algirdas Toleikis, Nida Siddiqui, Robert A. Cross, and Anne Straube (2020)
Activation of cytoplasmic dynein through microtubule crossbridging.
PREPRINT bioRxiv 2020.04.13.038950
doi: 10.1101/2020.04.13.038950. [Link]
This preprint has been highlighted by a prelight.
 synergy of microtubule-targeting drugs
synergy of microtubule-targeting drugs
The screening team at the Beatson Institute identified Mebendazole as a candidate to act in synergy with Docetaxel. We contributed experiments in RPE1 and PC3 cells treated with each of the drugs individually and together to show how they affect microtubule assembly and mitotic progression. The synergy of both drugs results frequently in mitotic catastrophe.
Linda Rushworth, Kay Hewit, Sophie Munnings-Tomes, Sukrut Somani, Daniel James, Emma Shanks, Christine Dufès, Anne Straube, Rachana Patel, and Hing Y Leung (2019)
Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment.
British Journal of Cancer.
doi: 10.1038/s41416-019-0681-5. [Link]
 phosphoregulation of microtubule binding
phosphoregulation of microtubule binding
Here we show that EML4 binds microtubules via the negatively charged E-hooks. Upon phosphorylation by mitotic kinases NEK6 and NEK7, EML4 binding to microtubules is reduced. This displacement of EML4 from microtubules reduces their stability. Dynamic microtubules are required for faithful chromosome segregation. Expressing non-phosphorylatable mutants of EML4 results in their association to microtubules throughout mitosis and spindle checkpoint activation and mitotic delay.
Adib R, Montgomery JM, Atherton J, O'Regan L, Richards MW, Straatman KR, Roth
D, Straube A, Bayliss R, Moores CA, Fry AM. (2019)
Mitotic phosphorylation by NEK6 and NEK7 reduces the microtubule affinity of EML4 to promote chromosome congression.
Sci Signal. 12(594):eaaw2939.
doi: 10.1126/scisignal.aaw2939.
[Link]
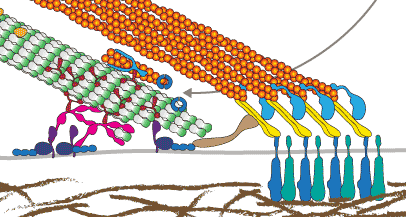 microtubules in cell migration
microtubules in cell migration
This is our contribution to the special issue on BIOCHEMICAL AND MECHANISTIC ASPECTS OF CELL MOTILITY AND MIGRATION. Our Essay summarises the functions of microtubules in cell migration including its role as directional transport tracks, signalling platforms and load-bearing elements.
Clare Garcin and Anne Straube (2019)
Microtubules in cell migration
Essays in Biochemistry 63(5): 509-520.
doi: 10.1042/EBC20190016
[Link]
 activating intracellular transport
activating intracellular transport
We show that contrary to other kinesin-3 motors, KIF1C is autoinhibited by an intramolecular interaction of its stalk with the microtubule binding surface of the motor domain. The phosphatase PTPN21 and the cargo adpater Hook3 activate KIF1C by binding to the stalk. Both activators increase the landing rate of the kinesin and are transported by the motor.
N. Siddiqui*, A.J. Zwetsloot*, A. Bachmann, D. Roth, H. Hussain, J. Brandt, I. Kaverina & A. Straube (2019)
PTPN21 and Hook3 relieve KIF1C autoinhibition and activate intracellular transport
Nature Communications, Volume 10, Article number: 2693
doi: 10.1038/s41467-019-10644-9
[Link to Paper]
Siddiqui,N., Bachmann, A., Zwetsloot,A., Hussain,H., Roth, D., Kaverina, I. and Straube, A. (2018)
Activation of intracellular transport by relieving KIF1C autoinhibition.
PREPRINT bioRxiv doi: 10.1101/488049
[Link to Preprint]
This preprint has been highlighted by a prelight.
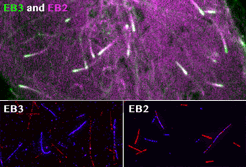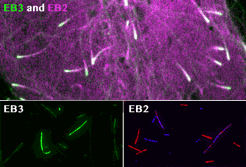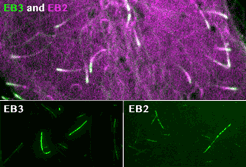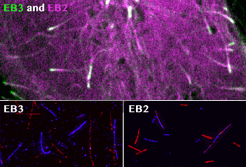 spatial EB positioning
spatial EB positioning
Our contribution to the special issue on reconstituting cell biology. We reconstituted the spatially distinct tip tracking of EB proteins in vitro and show that EBs sense the nucleotide state of two the beta-tubulins adjacent to their binding site. EB2 has a different nucleotide preference and prefers binding to mixed nucleotide lattices, while EB1 and EB3 need an environment of pure GTPgS to bind efficiently.
Roth, D., Fitton, B.P., Chmel, N., Wasiluk, N. and Straube, A. (2018)
Spatial positioning of EB family proteins at microtubule tips involves distinct nucleotide-dependent binding properties
Journal of Cell Science 132(4): jcs219550
doi: 10.1242/jcs.219550
[link to full pdf]
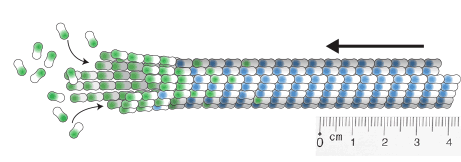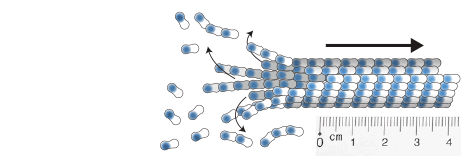 measuring microtubule dynamics
measuring microtubule dynamics
Our essay on how to measure microtubule dynamics.
Zwetsloot, A.J., Tut, G. and Straube, A. (2018)
Measuring microtubule dynamics
Essays in Biochemistry 62(6):725-735.
doi: 10.1042/EBC20180035
[link to open access article]
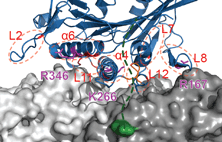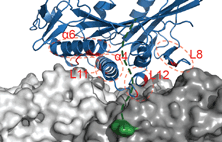 kinesin-3 review
kinesin-3 review
Nida and Anne review kinesin-3 transporters, their structural characteristics, intracellular cargoes and their regulation, with particular emphasis on the autoinhibited state and mechanisms of relieving autoinhibition by cargo and adapter proteins.
Siddiqui, N. and Straube, A. (2017)
Intracellular cargo transport by kinesin-3 motors
Biochemistry (Moscow), Vol. 82, No. 7, pp. 803–815
Russian version: Biokhimiya, 2017, Vol. 82, No. 7, pp. 1047–1062
[link to open access article]
 microtubules regulating cell migration
microtubules regulating cell migration
A comprehensive book chapter in "The Microtubule Cytoskeleton" exploring the diverse functions of microtubules in cell migration and neuronal pathfinding. Co-authored with Ulrike Theisen who is studying neuronal migration in zebrafish in Braunschweig.
Ulrike Theisen and Anne Straube (2016)
Microtubules Regulate Cell Migration and Neuronal Pathfinding
in: The Microtubule Cytoskeleton, Jens Lüders (ed), pages 151-189 DOI: 10.1007/978-3-7091-1903-7_6
[Link]
 encyclopedic microtubules
encyclopedic microtubules
Anne's contribution to a major textbook with 285 chapters. My chapter contains all you ever wanted to know about microtubules and the proteins that bind and regulate them in a nutshell.
Straube, A. (2015)
Microtubules and Microtubule-Associated Proteins (MAPs)
Encyclopedia of Cell Biology, Volume 2: Organizational Cell Biology, 2016, Pages 539-547
[link] [request pdf]
 wobbly phages as nanosensors
wobbly phages as nanosensors
This paper demonstrates the use of a fluorescently-labelled and surface-anchored bacteriophage M13 to measure wall shear stress. Bacteriophage anchored on collagen-coated slides and the surface of endothelial cells were subjected to various levels of shear stress by modulating buffer flow through the imaging chamber.
Lobo, D.P. ,Wemyss, A.M., Smith, D.J., Straube, A., Betteridge, K.B., Salmon, A.H.J., Foster, R.R., Elhegni, H.E., Satchell, S.C., Little, H.A., Pacheco-Gomez, R., Simmons, M.J., Hicks, M.R., Bates, D.O., Rodger, A., Dafforn, T.R., & Arkill, K.P. (2015)
Direct detection and measurement of wall shear stress using a filamentous bio-nanoparticle.
Nano Research, doi: 10.1007/s12274-015-0831-x
[link]
 chaperoning the spindle
chaperoning the spindle
We describe Nek6-dependent spindle recruitment of Hsp70, which is required for K-fibre stability and function. Data generated in the Straube lab suggest that the weak direct interaction of Hsp70 with microtubules is not affected by the Nek6-mediated phosphorylation at T66. Thus Hsp70 possibly acts by recruiting the chTOG/TACC3/Clathrin complex to spindle microtubules.
O'Regan, L., Sampson, J., Richards, M.W., Knebel, A., Roth, D., Hood, F.E., Straube, A., Royle, S.J., Bayliss, R. & Fry, A.M. (2015)
Hsp72 is targeted to the mitotic spindle by Nek6 to promote K-fiber assembly and mitotic progression.
The Journal of Cell Biology, 209: 349-358. doi:10.1083/jcb.201409151
[link]
 zippering microtubules
zippering microtubules
Here, we identify a previously uncharacterised isoform of microtubule-associated protein MAP4, oMAP4, as a microtubule organising factor that is crucial for myogenesis. Depletion of oMAP4 impairs cell elongation and cell-cell fusion. oMAP4 is required for paraxial microtubule organisation in muscle cells and prevents dynein- and kinesin-driven microtubule-microtubule sliding. Purified oMAP4 aligns dynamic microtubules into antiparallel bundles that withstand motor forces in vitro.
Mogessie, B., Roth, D., Rahil, Z. and Straube, A. (2015)
A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation
eLife, 4: e05697. doi:10.7554/eLife.05697.
[link] | [PDF]
 microtubule binding motif revealed
microtubule binding motif revealed
In this paper we identify the MT-binding domain in EML proteins. Daniel and Anne performed TIRF-based MT binding assays using full-length, mutant and truncation constructs of human EML1 fused to YFP. We show that EML contains a trimerisation domain that is required, but not sufficient for MT binding.
Richards, M.W., O'Regan, L., Roth, D., Montgomery, J.M., Straube, A., Fry, A.M. and Bayliss, R. (2015)
Microtubule association of EML proteins and the EML4-ALK variant 3 oncoprotein require an N-terminal trimerization domain
Biochemical Journal, 467: 529-536. doi:10.1042/BJ20150039
[link]
 transport on the go
transport on the go
Alice and Anne review the literature implicating kinesins in cell migration. It turns out we know surprisingly little about which kinesin transports which cargo to support cell polarity and directed migration. What we do know, you'll find in here.
Bachmann, A. and Straube, A. (2015)
Kinesins in cell migration
Biochem Soc Trans, 43(1): 79-83.
[PDF] | [pubmed]
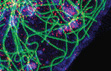 kinesin under MAP control
kinesin under MAP control
Here we show that the kinesin KIF1C is required for the de-novo formation of podosomes in vascular smooth muscle cells. We describe a pathway whereby activation of PKC results in the enrichment of CLASPs at microtubule ends, which stimulates KIF1C translocation to the cell periphery, where KIF1C localises to incipient podosome sites.
Efimova, N., Grimaldi, A., Bachmann, A., Frye, K., Zhu, X., Feoktistov, A., Straube, A. and Kaverina, I. (2014)
Podosome-regulating kinesin KIF1C translocates to the cell periphery in a CLASP-dependent manner.
Journal of Cell Science 127: 5179-5188. doi:10.1242/jcs.149633
[link]
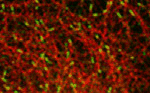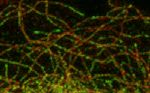 tip tracking complications
tip tracking complications
In this paper we demonstrate that CLASPs modify the EB binding site at the microtubule lattice. Ben and Daniel demonstrate the removal of the EB binding site from the microtubule lattice through CLASPs using TIRF-based in vitro reconstitution experiments.
Grimaldi, A.D., Maki, T., Fitton, B.P., Roth, D., Yampolsky, D., Davidson, M.W., Svitkina, T., Straube, A., Hayashi, I. and Kaverina, I. (2014)
CLASPs are required for proper microtubule localization of end-binding proteins.
Developmental Cell, 30, 343-352.
[link]
 exploring shapes
exploring shapes
In this paper we describe a computational framework to analyse and classify cell morphology. The approach uses a machine learning process based on the calculation of a shape similarity matrix and low-dimensional representation of cell shapes using diffusion map. We show that this approach is suitable for the highly variable cell shapes observed in migrating epithelial cells.
Jefferyes, S.D.R., Epstein, D.B.A., Straube, A., Rajpoot, N.M. (2013)
A novel framework for exploratory analysis of highly variable morphology of migrating epithelial cells.
Conf Proc IEEE Eng Med Biol Soc. 2013 Jul;2013:3463-3466, doi:10.1109/EMBC.2013.6610287
[link] [pubmed abstract]
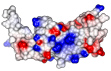 composite microtubule binders
composite microtubule binders
In this paper we demonstrate that TACC3 and clathrin form a composite microtubule binding site. Daniel and Anne did in vitro microtubule binding experiments of TACC3 and clathrin in the presence of AuroraA kinase to demonstrate the phosphorylation-dependent binding of the complex to microtubules.
Hood, F.E., Williams, S.J., Burgess, S.G., Richards, M.W., Roth, D., Straube, A., Pfuhl, M., Bayliss, R. & Royle, S.J. (2013)
Coordination of adjacent domains mediates TACC3–ch-TOG–clathrin assembly and mitotic spindle binding.
J. Cell Biol. 202:463-78
[link]
 microtubule rigidity
microtubule rigidity
In this paper we investigate the flexural rigidity of microtubules that have been stabilised by Taxol, non-hydrolysable GTP analogs or two different microtubule-associated proteins, tau and MAP4. We find that Taxol and GTPγS make microtubules more flexible, while tau and GMP-CPP make them stiffer. We show that combinations of these stabilisers do not have additive effects, but rather one stabiliser dominates the mechanical properties. MAP4 is not changing rigidity per se, but limits the variability of rigidity between microtubules.
Hawkins T.L., Sept D., Mogessie B., Straube A. and Ross J.L. (2013)
Mechanical Properties of Doubly Stabilized Microtubule Filaments.
Biophysical Journal 104: 1517-1528
[link to pdf] [pubmed abstract][request pdf]
>> see all publications here


 Research summary
Research summary Anne Straube
Anne Straube  Daniel Roth
Daniel Roth  David Corcoran
David Corcoran  Farha Naaz
Farha Naaz  Shivani Yadav
Shivani Yadav  Katie Brooks
Katie Brooks  Apolena Zounarova
Apolena Zounarova  Kate Mullarkey
Kate Mullarkey  Rowan Boustred
Rowan Boustred 
 Lokesh Kumar
Lokesh Kumar  Clare Garcin
Clare Garcin  Paula Esquivias
Paula Esquivias  Helena Coker
Helena Coker  Jack Chen
Jack Chen  Nida Siddiqui
Nida Siddiqui  Manas Chakraborty
Manas Chakraborty  Mohammed Abdelsamea
Mohammed Abdelsamea  Gaëlle Letort
Gaëlle Letort  Rose Gostner
Rose Gostner  Ulrike Theisen
Ulrike Theisen  Mike Downey
Mike Downey 
 Jessica Bithell
Jessica Bithell  Mahir Taher
Mahir Taher  Sareeta Bagri
Sareeta Bagri  Lewis Mosby
Lewis Mosby  Alexander Zwetsloot
Alexander Zwetsloot  Jonathan Brandt
Jonathan Brandt  Gokhan Tut
Gokhan Tut  Alice Bachmann
Alice Bachmann  Ben Fitton
Ben Fitton  Sam Jefferyes
Sam Jefferyes  Binyam Mogessie
Binyam Mogessie 
 Olivier Tardy
Olivier Tardy  Isaac Gardiner
Isaac Gardiner  Hannah Gay
Hannah Gay  Ana Clark (now Wallis)
Ana Clark (now Wallis)  Zoe Redshaw
Zoe Redshaw 
 Cyntia Fernandez Cuesta
Cyntia Fernandez Cuesta Christian Clarke
Christian Clarke Hannah Smith
Hannah Smith Alice Haworth
Alice Haworth Ainur Kakpenova
Ainur Kakpenova  Jacopo Credi
Jacopo Credi  Alina Finch
Alina Finch Alexandra Matthews
Alexandra Matthews Justyna Szyroka
Justyna Szyroka  Diana Sifuentes Munch
Diana Sifuentes Munch  Jessica Talbot
Jessica Talbot  Lewis Baker
Lewis Baker  Scott Clarke
Scott Clarke  Rozita Adib
Rozita Adib  Harriet Bell
Harriet Bell  Nikita Nicholls
Nikita Nicholls  Sarah Cosgriff
Sarah Cosgriff  Robert Lockley
Robert Lockley  Charlotte Carroll
Charlotte Carroll  Harold Moyse
Harold Moyse  Rachel Sheldon
Rachel Sheldon  Ali Rasooli-Nejad
Ali Rasooli-Nejad

 Ashvini Wijayapala
Ashvini Wijayapala  Pauline Chandon
Pauline Chandon  Molly McAinsh
Molly McAinsh  Alice Hayward-Wills
Alice Hayward-Wills  Hazel Hassan
Hazel Hassan  Max Phippen
Max Phippen  Aida Hassan
Aida Hassan  Julia Schander
Julia Schander  Samia Mohammed
Samia Mohammed  Elly Straube
Elly Straube  Meghana Kumar
Meghana Kumar  Luke Edwards
Luke Edwards  Zainab Rahil
Zainab Rahil  Natalia Wasiluk
Natalia Wasiluk  Agnieszka Skalecka
Agnieszka Skalecka 
 Andrew Carter
Andrew Carter  Andrew McAinsh
Andrew McAinsh  Jenny Ross
Jenny Ross 
 Andrew Fry and Richard Bayliss
Andrew Fry and Richard Bayliss  Irina Kaverina
Irina Kaverina  Ekkehard Straube
Ekkehard Straube  Nasir Rajpoot
Nasir Rajpoot  Steve Royle
Steve Royle